In short
Laboratory-acquired infections (LAIs) refer to human infections, acquired in a bio-containment facility (also known as laboratory), while working with pathogenic biological agents (i.e. the agents responsible for diseases such as bacteria, viruses, parasites, etc.). LAIs may at first affect staff working at the biocontainment facility, but can afterwards also become a broader public health concern if an infected staff member transmits the biological agent to the public and/or the environment. Monitoring of and timely reaction to LAIs are therefore important elements of preventing (public) harm following an unintentional infection. Hence, Sciensano in collaboration with Perseus BVBA investigated how monitoring of LAIs happens and is experienced in Europe, Canada and the USA. We also introduced a model that could achieve a more optimal monitoring of LAIs.
Project description
Laboratory-acquired infections (LAIs) refers to all direct or indirect human infections, regardless of whether they are symptomatic or asymptomatic, following occupational exposure to biological agents in a bio-containment facility. This study aimed at providing insight in the legal options for specific preventive measures (in particular vaccination and exclusion of candidate employees) and LAI monitoring, as well as their value and feasibility.
The monitoring of LAIs aims at:
- (rapid) detection of the occurrence of an adverse event with risk of human infection.
- prevention of consequences for human health and the environment
- creation of evidence in effectiveness of the required containment measures (evidence-based biosafety).
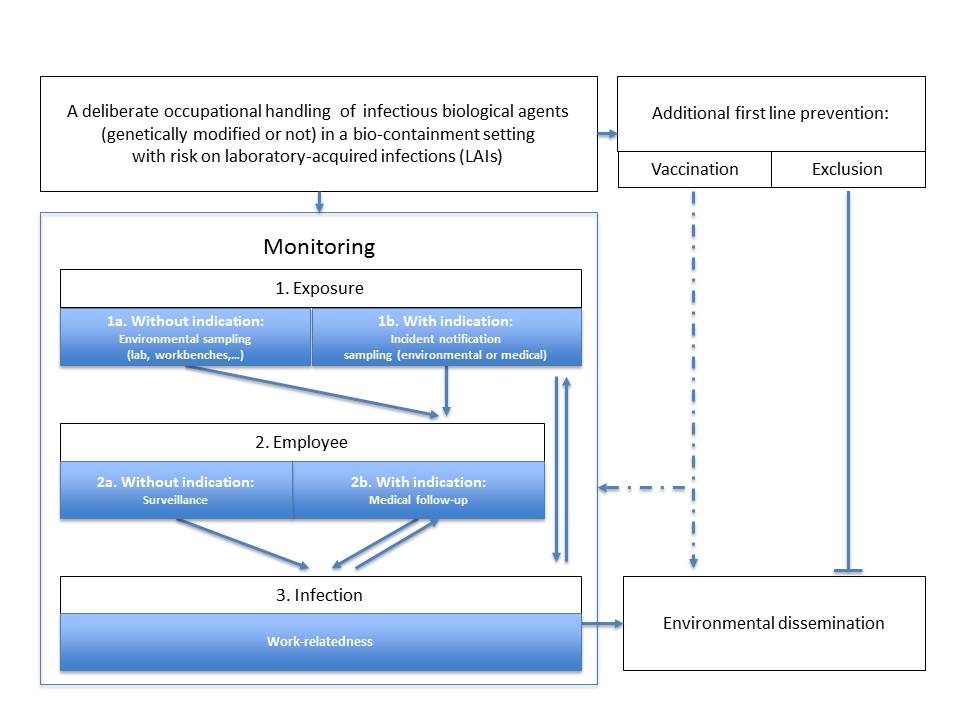
In the project, a conceptual model has been developed to position different monitoring levels, as stated in the image below:
- potential exposure
- the employee who is exposed to biological agents
- the occupational infection, thereby possibly starting with or without the indication of an exposure (e.g. bio-incident).
Results
The key findings of the project are that:
- directives 2009/41/EC and 2000/54/EC are complementary and provide for monitoring possibilities
- monitoring of LAIs could lead to more knowledge of risks and better management
- although legislation derived from Directives 2009/41/EC and 2000/54/EC is complementary, distinct government authorities might be involved in their implementation, causing fragmentation of authority. As a result notification obligations and follow-up might be overlooked
- the potential for adverse biological effects from activities with biological agents, including GMOs, for the environment and population:
- is considered low, based on the limited data, thus it is socially not a priority
- cannot be completely ruled out, mainly caused by non-GMOs from R&D (and relatively less from diagnotics activities)
- the share of GMOs is marginal as GMOs are often intrinsically contained with regard to the risk for the population and the environment (e.g. auxotrophy, nonreplicative, attenuation, …)
- there is no centralised reporting system for LAIs and accidents involving biological agents
- there is only a limited number of ‘lessons learnt’, which leads to
- incomplete and fragmented data on LAIs
- no optimal risk management for avoiding similar accidents and the pragmatic organisation of the legal requirements.
This project was commissioned by COGEM. The contents are the sole responsibility of the authors and may in no way be taken to represent the views of COGEM.
Sciensano's project investigator(s):
Service(s) working on this project
Partners




